Chapter 2 - Rocks and Minerals |
| This chapter is an introduction to rocks and minerals, but focuses primarily on minerals (various kinds of rocks are discussed in subsequent chapters). Minerals are the natural components that make up most solid materials found in the natural environment. Minerals are the inorganic crystalline compounds found in rocks, sediment, and soil. Everything solid in the landscapes around us are made of chemical compounds that have testable and identifying characteristics, allowing them to be classified. Minerals form in many different ways and often have unique, identifiable characteristics. Minerals are the basic components of rocks. How rocks form and where they occur are discussed in subsequent chapters. |
What are the most abundant elements in Earth's physical environment?The most abundant elements in our physical environment are: H, C, N, O, Na, Mg, Al, Si, P, S, Cl, K, Ca, FeSee their locations on the Periodic Table, Figure 2-3.) In chronological order by atomic number, these symbols are H hydrogen, C carbon, N nitrogen, O oxygen, Na sodium, Mg magnesium, Al aluminum, Si silicon, P phosphorus, S sulfur, Cl chlorine, K potassium, Ca calcium, and Fe iron. Be prepared to name these elemental symbols! These elemental symbols are used in discussions below and throughout the following chapters. These elements are: * ingredients of common minerals, rocks, sediments, and soil (solids) * components of seawater and air (liquids & gases) * essential nutrients for life (organic compounds). What is the elemental composition of the Earth's crust?Rock samples collected from around the world show that the chemical composition of the Earth's crust is not uniform, but certain elements are much more abundant than others (Figure 2-4). The elemental composition of the Earth's crust, oceans, and atmosphere are directly linked to the processes that formed the planet within the Solar System billions of years ago (as discussed in Chapter 1). |
 Fig. 2-3. Most abundant elements in the physical environment. 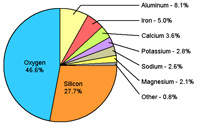 Fig. 2-4. Elemental composition of the Earth's solid crust. |
The Rock CycleThe rock cycle is a graphic and conceptual model used to illustrate common rocks and earth materials and the processes that form or change in the Earth’s crust over time (Figure 2-7). The rock cycle depicts the series of events in which a rock of one type is converted to one or more other types and then back to the original type (see the general classification of earth materials below). Both products (rocks and sediments) and processes (such as melting, cooling, erosion, deposition, metamorphism, remelting) are part of this idealized cycle. The passage of geologic time is the essential component, although some processes are much faster than others. Note that all these types of processes are taking place simultaneously, but at different locations on and within the crust. It is important to note that rock cycle processes occur on other rocky planets or moons but rates may vary due to the presence (or lack of) atmospheric gases or fluids (including water) or availability of heat enough to melt rocks. The rock cycle and basic geologic principles are discussed in Chapter 3. |
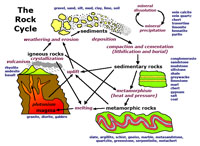 Fig. 2-7. The Rock Cycle is a conceptual model that portrays processes and products changing over time |
Rocks are classified into three general types based on their geologic origin.Rocks are classified into three general groups: igneous, sedimentary, and metamorphic.The term igneous applies to rocks or minerals that solidified (crystallized) from molten or partly molten material—referring to magma (molten material underground), or lava (molten material on the surface of a volcano) (Figure 2-8). The word igneous also applies to the processes related to the formation of such rocks. (Igneous rocks and volcanism are the focus of Chapter 8). The term sedimentary applies to materials consisting of sediments or formed by deposition. The word sedimentary applies to both the processes and the products of deposition. Sedimentary rocks form from sediments (Figures 2-9 and 2-10). (Sediments are discussed in Chapter 9; Sedimentary rocks and processes are the focus of Chapter 10.) The term metamorphic pertains to the process of metamorphism or to its results. Metamorphism is the chemical, mineralogical, and structural adjustment of solid rocks to changing physical and chemical conditions imposed at depth below the surface and below surficial zones where sedimentary processes take place (Figure 2-11)). (Metamorphic rocks and processes are the focus of Chapter 11.) In summary, characteristics of rocks include: 1) Rocks may be composed of a single type of mineral, or may be a mixture of minerals. 2) Rocks may also have organic residue (non-mineral) components. 3) Rocks preserve evidence of the physical environments in which they form. |
Learn more about gems and minerals at the Gemological Institute of AmericaIf you live in the San Diego area, consider taking a guided tour at the Gemological Institute of America (GIA) in Carlsbad, CA. The GIA offers tours of their facilities where students and professionals are taught how to identify, evaluate, and work with gems and precious stones. The main building at GIA Headquarters has hallways lined with exhibits filled with exceptional examples of gems and provide discussions about their nature and occurrence. The GIA a large hall with special exhibits, and a room with one of the best mineral and gem-bearing rock exhibits in the world. The tour is free, but must be scheduled by reservation in advance. GIA is an excellent place to learn more about gems and minerals. Note that San Diego County has a long history of gem mining, and GIA provides a lot of information about historic mining operations with spectacular examples of local tourmaline, morganite, and other gem minerals. |
Crystalline vs. Non Crystalline SubstancesAs stated above, a crystal is a piece of a homogeneous solid substance having a naturally geometrically regular form with symmetrically arranged plane faces. Note that this geometric arrangement occurs on an atomic level (too small to see even with a powerful microscope), but this basic atomic arrangement repeats itself many trillions of times to form a single crystal grain.A crystalline substance has the structure and form of a crystal or is composed of crystals. In our world there are many crystalline substances. To illustrate, let's start with salt (chemical formula - NaCl, or sodium chloride) or as geologists call it, the mineral halite. Salt usually precipitated from evaporating water without organic processes and is thus a mineral. Halite is a very soft mineral because it's elements, sodium and chlorine are held together by ionic bonds. Sugar (C6H12O6, sucrose) also forms crystals when precipitated from water, but because it is organic and therefore it is not a mineral. Sugar sold as rock candy has a mineral like appearance because it has a crystalline form, but it is not a mineral. Very few things that are solid are not crystalline. However, because in our world much of what we see is formed by life processes, most observed solids are not minerals. You will quickly argue that rocks are all around us and that they are made of minerals, however in terms of variety only about a dozen minerals (the rock forming minerals) are abundant, and in fact there is a great deal more variety of organic solids around us than minerals. We rarely spend much time observing minerals and really the average beginning student knows almost nothing about them and their properties. Figure 2-13 shows an organized mineral structure with an ordered arrangement of atoms (considered crystalline) and a disorganized substance without a crystal structure (considered non crystalline). Both can be solid, but a disorganized solid is called non-crystalline or amorphous. Both are held together by chemical bonds, but crystalline solids have an ordered structure that fills space in 3 dimensions. With a crystalline structure you can predict where the next atom can be found in the crystal structure. Of the few inorganic, non-crystalline solids dealt with in gemology, glass is the most important. Glass is an amorphous substance that has no orderly arrangement of atoms (it is non crystalline). Glass is also a mixture of chemical compounds. Glass forms by rapid cooling of substances that have been melted to a liquid. There are natural and man-made glasses. Man made glass is often used as a gem substitute, commonly as costume jewelry. |
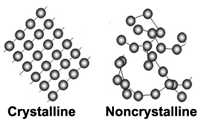 Fig. 2-13. Minerals are made up of atoms arranged in a crystalline structure. The crystals may range in size from on a microscopic scale to full-sized visible masses. Non crystalline (amorphous) substances (like glass) have no orderly arrangement of atoms. |
Atomic Structure of Crystals |
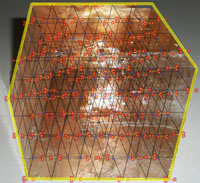
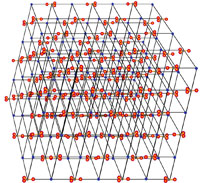
| Minerals are chemical substances composed of atoms arranged in unique crystal structures. A crystal structure describes a highly ordered repeatable arrangement of atoms. Note that there is an important difference between the chemical formula of a mineral and the molecular crystal structure of a mineral! A chemical formula is only a description of the elements that make up a pure chemical compound. Only when molecules are arranged in an orderly, repeatable symmetric pattern will it be considered a mineral. For instance, water (H2O) is not a mineral, but ice is! Unit Cells In CrystalsA crystal structure can be thought of as an infinitely repeating array of three-dimensional boxes known as unit-cells. The unit cell is calculated from the smallest and simplest possible representation of molecules arranged to form a repeating crystal structure. Crystals consist of repeating unit cells ranging from the atomic level to consisting of many quadrillions of unit cells combined together in observably visible crystals (see example with halite below).A simple way to illustrate the arrangement of atoms into a geometric crystal structure is to use marbles stacked in different ways (Figures 2-14 and 2-15); these forms shown ins how when marbles are stacked in different ways they can illustrated the simplest forms of different crystal arrangements. Figure 2-14 illustrates vertically stacked marbles, whereas Figure 2-15 shows marbles stacked in an offset arrangement that is the most tightest possible with spheres of uniform size. Figure 2-14 shows cubic and rectangular cuboids, and octagons (double pyramid) forms. Figure 2-15 shows hexagonal prism and pyramidal forms. In both illustrations, the marbles are the same size, only the stacking arrangement is different. Note the unit cell arrangement for each of the 4 forms. |
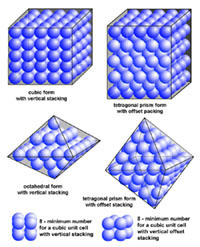 Fig. 2-14. Vertically stacked marbles illustrate atomic arrangement of crystal forms. Cubic, rectangular cuboids, and octahedral forms can form from the two arrangement of marbles. Note that a minimum of 8 marbles represents the minimum unit cell to create the larger vertical stacked and offset vertically stacked cubic structures. |
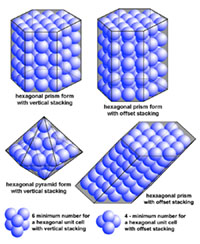 Fig. 2-15. The same arrangement of stacked, offset marbles can produce hexagonal crystal forms (prisms and pyramids). Add more layers of marbles (atoms) and the crystal grows larger. Note only 6 marbles are needed for vertical hexagonal unit cell, and 4 marbles are needed for a offset-stacking hexagonal unit cell. |
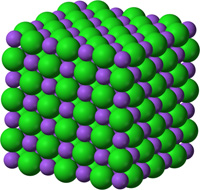
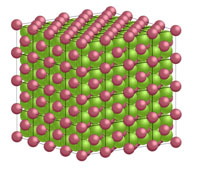
Common and Important Minerals IllustratedThe discussions figures below illustrates the crystal structures of common or important minerals. Note that the arrangement of atoms in the mineral crystal structures illustrate below are magnified and expanded many millions of times from how they may appear on a molecular scale. Fortunately, scientists over the centuries have developed many tools for figuring out ways to indirectly see and interpret the microscopic crystal structure of minerals! Important rock forming minerals are illustrated below.Halite (common table salt)Figure 2-16 shows halite (or common table salt, NaCl) which consists of two elements sodium (Na) and chlorine (Cl) that when combined in a repeating arrangement in a crystalline structure (see Figure 2-17). Note that it would take 4 NaCl molecules (4 sodium atom and 4 chlorine atoms) to make of the minimum cubic unit cell representing the crystal structure of halite. The arrangement of atoms in a cubic structure of the mineral, halite, is repeatable whether on an atomic scale or a microscopic scale (as in table salt) or macroscopic (fist-sized chunk)(Figure 2-18). Halite crystals grow from precipitating from water and is manufactured worldwide by evaporating seawater (see Figure 2-19 as an example where and how it is done using evaporation ponds in arid settings). |
|
|
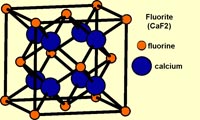
FluoriteFigure 2-20 shows the crystalline structure of fluorite. Although the chemical formula of fluorite is CaF. However, it takes eight atoms of calcium (Ca) and sixteen atoms fluorine (F) are needed to make the minimum-sized unit cell of the crystal structure of mineral fluorite (Figure 2-21). Billions of unit cells are required to combine to make a single small crystal you can hold in your hand! The geometric arrangements of unit cells on an atomic scale determine how a crystal appears on a macroscopic (visible) scale (Figure 2-22). Because minerals have repeating geometric arrangement of atoms in crystal lattices, crystals can be fashioned into a variety of shapes that are compatible with the crystal structure. In the case of fluorite, which usually exists in cubic crystals, it can be split and shaped into octahedral shaped crystal specimens (commonly sold in rock shops)(see Figure 2-23). The arrangement of molecules within a crystal structure determines how a mineral crystal can be split and cut into geometric shapes, including shapes used in finished gemstones (as illustrated in Figures 2-12). It is important to note that in most cases, the shape of a fashioned gemstone is nothing like the shape of a natural mineral crystal shape as they appear in nature. A gemologist cutting gemstones will closely examine the crystal structure of a mineral before faceting it into a gemstone. |
|
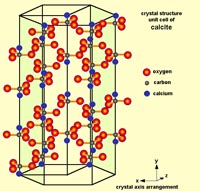
Carbonate Minerals: Calcite, Aragonite, and DolomiteCarbonate minerals have carbonate ions (-1CO3) within their mineral structure. In nature, most carbonate minerals form from the interactions of carbon dioxide and metals dissolved in water.The mineral calcite is perhaps the most amazing mineral. It has many crystalline forms and can form in many geologic settings. It is also an exceeding important mineral resource - it is used in the manufacture of cement, and is used in some manner in the process of manufacturing of thousands of compounds used in industry, including the manufacture of steel and the production of medicines and food. Calcite consists of a crystalline structure composed of molecules of calcium carbonate (CaCO3). Basically, the calcium (Ca) comes from the Earth, and the CO3 comes from the atmosphere, and nearly all the CaCO3 is deposited by biological activity in the oceans and precipitated from water underground. It is important to note that CaCO3 is a chemical formula only represents a single molecule. It takes many molecules of CaCO3 to make the unit cell of pure mineral calcite (see Figure 2-24). A pure specimen of calcite (CaCO3) would be perfectly clear form called Iceland spar (discussed below with Fig. 2-53). With pure calcite the unit cells will have 28 molecules of CaCO3, however, there can be a variety of other elements that can be substituted for a few of the calcium and carbon atoms with a unit cell, and it will keep the general crystal pattern of calcite. Elements including sodium, magnesium, iron, zinc, chromium, strontium, barium, and sulfur and can sneak into the structure of the unit cell and still maintain the general character of crystalline calcite. However, these differences can result in varieties calcite with some subtle differences in physical properties including color, crystal form, and special properties including fluorescence, phosphorescence, and thermoluminescence (discussed below). Calcite also doesn't fit the definition of a true mineral because it can also be of biological origin—a product of respiration, excretion, and precipitated or incorporated into the skeletal structures in plankton, microbial deposits, algal and coral reefs, and incorporated tissue of plants, invertebrate shells, and the shell of eggs. The arrangement of unit cells can produce differently shaped crystals. For example, calcite can form several variation including dogtooth spar, nail-head spar, and other combined forms of these crystal varieties (see Figures 2-24 to 2-26). Note that each of the crystal forms in Figure 2-25 have a hexagonal shape. This variation of crystal shapes is related to the physical conditions of where the mineral formed. Also important to note is that calcite also has an internal molecular arrangement that has a rhombohedral crystal form. This is because there are weaker bonds within the crystal structure that allow crystals to split along cleavage plains. Figures 2-27 to 2-29 show how the molecular arrangement of atoms (Ca, C, and O) give rise to the crystal structure of calcite that allows it to be split along cleavage plains. When a large crystal of calcite is crushed, all the fragments, even down to a microscopic level, will display the rhombohedral cleavage shape pattern. |
|
| Aragonite is another mineral composed of calcium carbonate (CaCO3) but has a different crystal structure and has different physical properties (Figure 2-30). Dolomite is another carbonate mineral. Dolomite often forms from calcite by the substitution of a magnesium atom with a calcium atom (Figure 2-21). Calcite has a hexagonal crystal structure, whereas aragonite has an orthorhombic crystal structure (see crystal systems below). Carbonate Rocks: Limestone and DolostoneCalcite is the dominant mineral in the sedimentary rock called limestone. Over time, groundwater rich in dissolved magnesium can seep through limestone, gradually converting calcite to dolomite. As a result, ancient limestone rock formations often contain higher concentrations of dolomite than calcite. If a rock has more that 50 percent dolomite it is called dolostone. |
 Figure 2-30. The mineral aragonite is also composed of calcium carbonate (CaCO3), but the molecules are in a different crystalline structural arrangement than calcite. |
 Figure 2-31. The mineral, dolomite, has a chemical formula of CaMg(CO3)2. It has a trigonal-rhombohedral crystal form. The pink color comes from traces of iron within the crystal structure. |
What Is Mineral Cleavage?Mineral cleavage is the tendency of crystalline materials to split along definite crystallographic structural planes (or, for clarification, to break along smooth planes parallel to zones of weak bonding in crystalline substances). For instance, as illustrated above in Figures 2-27 to 2-29, calcium carbonate forms crystalline forms, calcite and aragonite. However, when a mineral sample of calcite is crushed, the crystals shatter along planes of weakness in the crystal lattice. In the case of calcite, the crystals break along 3 planes of weakness within the crystal structure, forming rhombohedral blocks. These cleavage planes are always at the same angles (in 3 directions, the x, y and z dimensional axes). The rhombohedral shape of the calcite crystal fragments are always the same, whether as a hand-size specimen or crystal fragments on a microscopic level. (The same is true for halite illustrated above in Figure 2-18, except the salt crystals are cubes instead of rhombs.) Mica minerals display exceptional mineral cleavage (including muscavite and biotite, shown in Figure 2-1). |
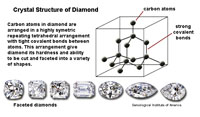
| Three factors play important roles in the physical properties of mineral: 1) the crystal structure, 2) character of chemical bonds within crystalline substances, and 3) the ability of substances to split along cleavage planes. How the arrangement of atoms affect physical properties is easily illustrated with two carbon minerals, graphite and diamond. In Figures 2-32 and 2-33, the lines between carbon atoms represent chemical bonds. The structure of minerals and their bonding results in the difference in the physical properties of the two minerals, for example hardness: graphite is one of the softest minerals (used in pencils) and diamonds are the hardest mineral, even though they are both composed of elemental carbon. These factors, particularly the hardness of a mineral and its tendency to split along cleavage planes, determine if and how a mineral specimen might be cut or faceted into a gemstone. |
 |
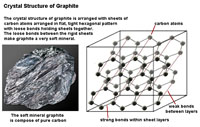 |
| Figure 2-32. Crystal structure of the mineral diamond. | Figure 2-33. Crystal structure of the mineral graphite. | |
|
||
How Many Crystal Shapes Are There?Well over 4,000 different minerals have been identified occurring naturally in the world. There are probably many more. Hundreds of thousand of inorganic compounds are known (and patented) and perhaps billions of organic compounds exist (having carbon and hydrogen and other elements combined in complex molecules). However, with all the chemical compounds that are known, there are only a relatively low number of naturally occurring, common or important mineral compounds that are gems or have economic significance. Figures 2-34 to 2-39 illustrate a classification of natural crystal forms and shapes (grouped within crystal systems). Minerals have characteristic crystal shapes that can be used to help identify them. |
|
Crystal Systems - Crystal Forms and Selected Crystal Shapes |
|
| Figure 2-34. Cubic and isometric crystal system Crystal forms: include cube, octahedron, dodecahedron, and other more complex forms. |
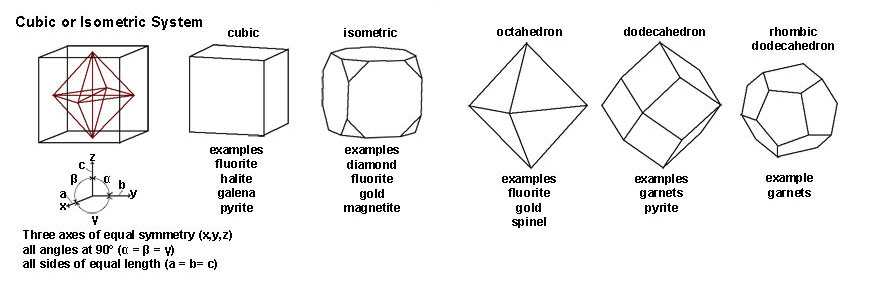 |
| The Cubic or Isometric System include all crystal shapes that have symmetry axes in equal lengths in 3 directions (at 90º angles to each other). Common minerals that have a cubic/isometric crystal form include halite, fluorite, galena, pyrite, magnetite. Gem minerals include diamond, garnets, spinel, and gold. | |
| Figure 2-35. Tetragonal crystal system. "Rectanguloid" shapes, prisms, pyramids, and complex forms. |
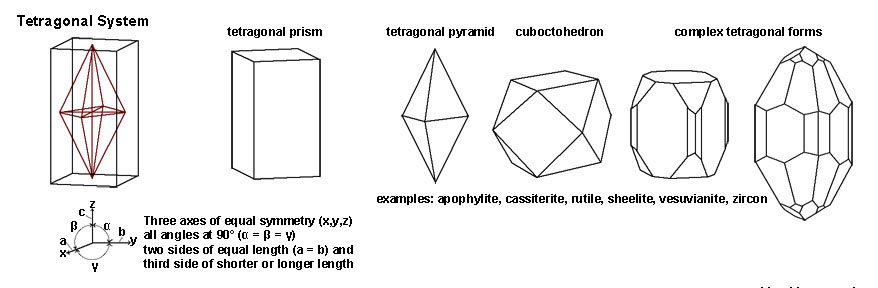 |
| The Tetragonal System includes all crystal shapes that have three axes of symmetry all at right angles (90º) of each other. However, two sides of the crystal axes share equal length, whereas the length of the third axis is either shorter or longer than the other two. Some examples of minerals include apophylite, cassiterite, sheelite, and vesuvianite. Gems include zircon and rutile. | |
Figure 2-36. |
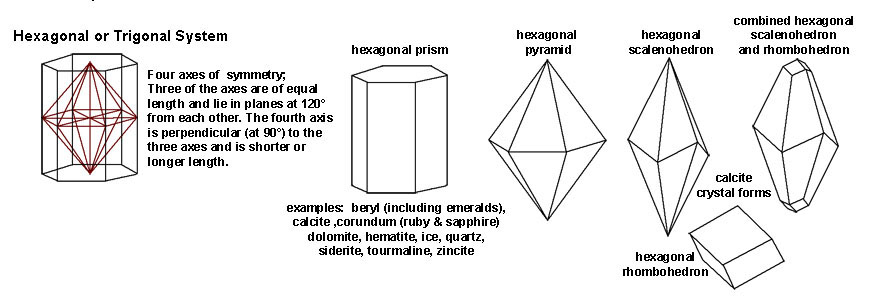 |
| The Hexagonal or Trigonal System includes crystal shape that are hexagonal. Three of the crystal axes are of equal length and lie in planes that are 120º from each other. The fourth axis is perpendicular (90º) to the three axes and is either shorter or longer to the other axes. Minerals with hexagonal form include calcite, dolomite, hematite, ice, quartz, and siderite. Gem minerals include beryl (including emerald), corundum (including ruby and sapphires), quartz varieties (crystal, citrine, amethyst), and tourmaline. | |
| Figure 2-37. Orthorhombic crystal system: prisms, pyramids, and combined forms. |
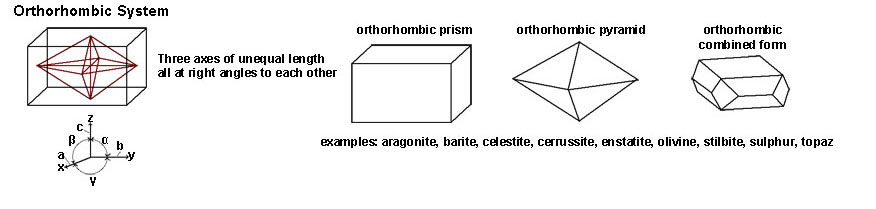 |
| The Orthorhombic System includes crystal shapes that have three axes of equal length but all at right angles (90º) of each other. Minerals with orthorhombic forms include aragonite, barite, celestite, cerrussite, enstatite, olivine, stilbite and sulfur. Gem minerals include peridote (olivine) and topaz. | |
| Figure 2-38. Monoclinic crystal system |
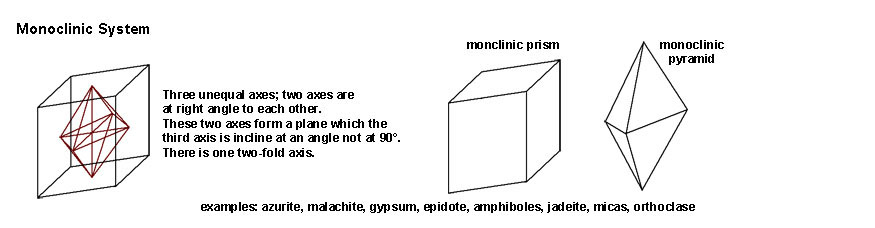 |
| The Monoclinic System includes crystal forms that have three unequal axes; two of the axes are at right angles (90º) but the third axis is inclined at an angle not at 90º. There is one two-fold axis of symmetry. Mineral examples include azurite, malachite, gypsum, epidote, amphiboles, jadeite, micas, and orthoclase. | |
| Figure 2-39. Triclinic crystal system |
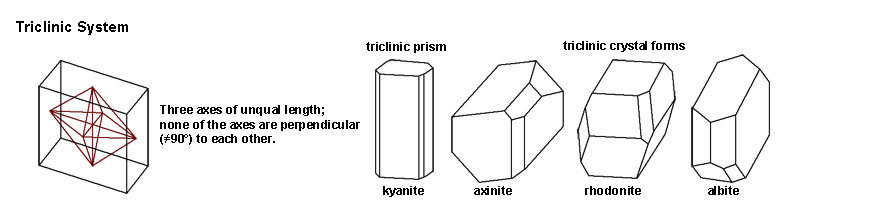 The Triclinic System includes crystal forms where the three axes are of unequal length, and one of the axes are perpendicular to each other. Mineral examples include kyanite, axinite, rhodonite, and albite. |
How can physical and chemical properties of minerals be used for their identification? |
||||||
| All minerals have unique properties that aide in their identification. Some minerals have unique characteristics that have an appearance or physical properties that make them easy to identify. However, these identifying characteristics may not be easy to determine without more extensive testing. Fortunately, the most common minerals are fairly easy to identify by their general appearance or with simple tests for hardness, crystal form, color, magnetism, and streak (Streak means it leaves a colored line when scratched on a piece of tile or hard surface). Note that some tests can be destructive to mineral samples (such as measuring hardness, streak, malleability, elasticity, and testing with acid). In addition, tasting a mineral is definitely not recommend - some are actually poisonous! Washing your hands after handling unknown mineral samples is always recommended. | ||||||
|
||||||
Observable Properties of MineralsThe following physical properties can be used to identify a mineral through sensory observations or conducting simple tests. Equipment for such tests are typically available in science education departments or are available from commercial sources. |
| Easily observable physical characteristics (simple visual observations of the form and character of some minerals) are illustrated below. |
| Crystal form—many minerals have unique and sometimes obvious crystal structures, however, crystal structure alone may not be enough to identify a mineral. For most samples used in mineral tests, crystal form may not be apparent or easily measurable. |
 |
| Fig. 2-40. Amazonite is a blue-green form of microcline feldspar. Samples of feldspars are fairly easy to find or purchase, and they typically have good crystal form (angles) for students to measure. |
| Color—some minerals have very distinct colors, however, color is not a reliable indicator by itself. |  |
| Fig. 2-41. Some minerals have obvious color associations. The combination of color with other mineral characteristics make the easy to identify: malachite (green), sulfur (yellow) and cinnabar (blood red). Problems arise with mineral samples are white or gray - there are dozens of minerals that have those neutral tones and make them difficult to easily identify without other tests. |
| Cleavage—the tendency of a crystallized substance to split along definite crystalline planes, yielding smooth surfaces. Mica, feldspar, calcite, and selenite gypsum have good mineral cleavage. Flat, smooth, shiny and reflective surfaces on specimens may be either crystal surfaces and/or cleavage. |  |
| Fig. 2-42. Many minerals have cleavage planes that make them easy to identify, with micas (biotite is black mica, muscovite is silvery-white mica) being perhaps the most easy to recognize. Crushing irregularly shaped samples may demonstrate repeatable shapes associated with cleavage planes. Common minerals that easily display cleavage patterns when crushed or broken include calcite and feldspars. |
| Striations—some mineral crystals have fine, narrowly-spaced lines on crystal surfaces. (Examples of minerals that may display striations include hornblende, pyrite and selenite (a crystalline form of gypsum). |
 |
| Fig. 2-43. Mineral crystals that grow in open cavities sometime display striations that are parallel to the crystal axes within the mineral's crystal structure. This sample shows a pyrite crystal with obvious striations. Note that striations may not occur on all all examples of a mineral. For example the cube-shaped pyrite specimen shown in Figure 2-44 does not display striations. |
Luster—the description of the quality and intensity (sheen or shine) of light reflected off of a mineral, particularly a reflective appearance of the exterior of crystal surfaces and cleavage planes. There are many kinds of luster:
|
 |
| Fig. 2-44. Pyrite (left) and galena (right) have a metallic luster. | |
 |
|
| Fig. 2-45. Tiger eye (a variety of quartz) displays chatoyancy luster. | |
 |
|
| Fig. 2-46. Labradorite (a variety of feldspar) displays a schiller luster. |
|
Optical Properties of MineralsTransparency—or more correctly, diaphaneity, is an evaluation of how light passes through a mineral, with general descriptions of being transparent (meaning clear enough for an object to be seen through a sample); translucent (a substance transmit light but it is dispersed or cloudy in appearance), or opaque (a substance will not transmit light). Few common minerals are transparent. Quartz and calcite can have high transparency (see Figures 2-51 and 2-53). Common milky quartz is typically translucent (light passes through but is diffuse, see Figure 2-52). |
 Fig. 2-51. Crystals of pure quartz are transparent like glass when clear. |
 Fig. 2-52. Milky quartz is translucent (cloudy, but allows light to be transmitted). |
| Double refraction—light passing through clear calcite (a variety called Iceland spar) will transmit a double image. Clear calcite can split a laser beam into two separate beams. Figure 2-53 shows a piece of Iceland spar causing the X pattern of the underlying paper to be doubled on itself. |  Fig. 2-53. Clear calcite displays double refraction. |
Fiber optic properties—a notable example is ulexite, a soft borate mineral moves images from one side of a cut sample to the other side with a cut surface. Figure 2-54 shows the X pattern on the underlying piece of paper transmitted to the surface of the ulexite sample. | 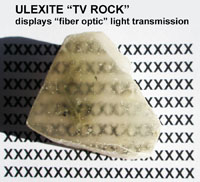 Fig. 2-54. Ulexite (also called "TV Rock") shows fiber-optic like properties. |
Non-Visual Sensory Characteristics of Minerals |
| Feel—The feel of a rock is not a reliable method of testing minerals, however certain minerals have textures like "soft, silky, satin, smooth, hard, heavy or light" - but these characteristics are poorly definable as a reliable means for identifying minerals. Odor—few minerals have an odor. Sulfur-bearing minerals may put off a rotten-egg like smell. Many rocks of sedimentary origin have the smell of petroleum. Taste—halite tastes like salt (because it is NaCl). (Note that tasting minerals and rocks is generally not recommended! Some minerals can be quite poisonous.) |
Simple Tests For Identifying Minerals |
| Minerals have a variety of physical and chemicals properties that can be evaluated using simple tests. The following tests are simple determinations using common laboratory equipment and supplies. Note that some of these are destructive to samples being tested! |
| Hardness—minerals have different durability properties. Depending on mineral chemistry and crystal structure, minerals have varying degrees of hardness. Simple tests of scratching mineral samples with items or material of known hardness can give a general range of hardness of a specimen. Note that minerals held together by ionic bonds (like halite, gypsum, and calcite) tend to be softer than minerals Mohs Hardness Scale is a list of hardness of common minerals (Figure 2-55) used in mineral testing laboratory exercises. Many gemstone have higher hardness. Diamonds are the hardest mineral, having a Mohs scale hardness of 10. Note that testing the hardness of minerals may be destructive to samples! |
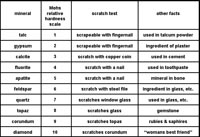 Fig. 55. Mohs Hardness Scale |
| Magnetism—iron (the natural mineral iron in crystalline form) and magnetite (Fe3O4) are common magnetic rocks, iron-rich meteorites are also magnetic. Figure 2-56 is sample of Diablo Canyon (Arizona) iron meteorite that is highly magnetic. Many minerals rich in iron are partly magnetic and display measurable magnetic susceptibility that can be useful for geophysical exploration. Large bodies of rock containing iron-rich minerals can be remotely detected below the earth surface, and may be useful for detecting hidden faults, water-filled sedimentary basins, or potentially economically valuable mineral resource deposits. Magnetic susceptibility measurement are used in regional geophysical mapping. |
 |
| Fig. 2-56. Magnets stick strongly to some iron minerals, such as native iron (naturally pure iron in mineral form) and the common mineral, magnetite. Other minerals show weak magnetic attraction including iron-rich metallic and metalloid minerals: hematite, goethite, chromite, franklinite, pyrrhotite, and siderite. Shown here, a magnet sticks strongly to a meteorite composed of the metallic iron-nickel mineral crystals (kamacite and taenite). |
| Hematite—A reddish, steel gray, or black mineral consisting of ferric oxide (Fe2O3). Limonite—An amorphous orange to brownish mineral consisting of a mixture of hydrated ferric oxides, important as an iron ore. Rust on iron vehicles is essentially limonite. Magnetite—a gray-black magnetic mineral that consists of iron oxide (Fe3O4) and is an important form of iron ore. Magnetite is highly magnetic. Pyrite—a brass-colored mineral, FeS2, occurring widely and used as an iron ore and in producing sulfur dioxide for sulfuric acid. Also called fool's gold, iron pyrites. |
 |
 |
| Fig. 2-57. Iron minerals: Hematite and Limonite | Fig. 2-58. Iron minerals: Magnetite and Pyrite |
| Specific gravity—a measure of the density of a mineral. Specific gravity is the ratio of the density of a substance to the density of water. Tests for specific gravity require some laboratory equipment. Specific gravity is a measure of weight with a known volume (Figure 2-59). | 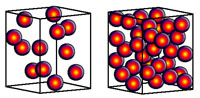 |
| Fig. 2-59. Two equal size cubes with dots representing atoms. The box on the left has fewer atoms in the same amount of space as the second box. The second box would therefore be denser than the first box. | |
| Streak—soft minerals may leave a streak of color on a piece of tile. Hematite makes a red streak, pyrite is brown, magnetite is black, etc. Be aware that streak tests can be destructive to mineral samples. |
| Fluorescence—some minerals glow colors under a blacklight including some fluorite, calcite, and zinc minerals. Different minerals glow brightly (fluoresce) under different wavelength of ultraviolet light, sometimes in different colors under different wavelengths. The crystal structures of fluorescent minerals allow ultraviolet energy to be absorbed and the energy is released in a visible color wavelength (see Figure 2-60). Most rocks and minerals are not fluorescent. Only a few common minerals will glow under common blacklight that releases long-wave ultraviolet light. Many others will glow under short-wave ultraviolet lamps (that are potentially hazardous to use improperly). Phosphorescence—some minerals absorb light energy and release light when the light is turned off. Some varieties of calcite, zinc minerals, and minerals rich in phosphorus sometimes display phosphorescence. Phosphorescence is only observable in a very dark setting - very shortly after energy source (visible light, or better, ultraviolet light) is shut off. In most cases, the phosphorescent glow ends quickly. Some phosphate-rich calcite and zinc minerals can glow for quite a some time after being exposed to a light source, with brightness decaying slowly over time. |
 normal light
normal light
|
 short-wave ultraviolet light |
| Fig. 2-60. Fluorescent minerals from Franklin, NJ. Under normal light and under short-wave UV light: calcite glows red, and wilmenite and other zinc-bearing minerals glow green. |
||
See Fluorescent Minerals of Southern California (see minerals under different wavelenghts of ultraviolet radiation) |
| Thermoluminescence—some minerals will glow in colors when heated, similar to a hot burner on a stove or an object held under a torch flame. Note that heating gems and minerals samples can (probably will) alter or destroy them. |
| Radioactivity— Radioactive elements that occur in rocks and minerals include potassium, thorium, radium, and uranium. and may display measurable radioactivity. Most mineral samples do not have measurable levels of radioactivity. However, many older collections in science departments may have radioactive mineral samples, and these should be clearly identified and not handled. Radiation, like magnetism and gravity, are used in geophysical mapping and resource exploration. Granitic rocks tend to be slightly more radioactive than other rocks having trace concentrations of uranium or thorium. Fossil wood from the Colorado Plateau region can sometimes be radioactive. It is advisable not to collect radioactive material because of the potential health risks. If collected, they should be clearly marked and stored in appropriate containers. They may be illegal to own or transported. |  |
| Figure 2-61. A geiger counter us used to measure materials for radioactivity. The sample shown here is a piece of gold ore from the Witwatersrand Gold Mine in South Africa. The gold is mixed in with uranium-bearing minerals and quartz. Many locations where gold occurs there may be other heavy elements, including uranium. |
| Acidic reaction—Calcite fizzes when exposed to mild acid, releasing carbon dioxide gas. Dolomite will fizz in hot acid. Note that all minerals are chemicals that can react to chemical agents, altering or destroying them. Whereas gemstones are typically durable, the can be susceptible to chemicals added to cleaning fluids. Iron-bearing mineral will react to oxidizing compounds like bleach. Be sure to check on appropriate cleaning agents before cleaning gemstones or gem-bearing jewelry |
 |
| Figure 2-62. Some minerals will react to exposure to acid. Calcite [CaCO3] fizzes when exposed to hydrochloric acid (HCl) or vinegar (acetic acid), , releasing carbon dioxide gas.. Dolomite [CaMg(CO3)2] will fizz only in hot acid. Note that acid will not only destroy mineral samples but can also ruin clothes! |
| Malleability—metals like gold, copper, iron, and silver is able to be hammered into objects. Elasticity—soft minerals may be bendable (like mica); most minerals fracture or shatter when put under stress or shock. |
 |
| Figure 2-63. Whereas it is sometime fun to smash things, it is not really a useful means of testing minerals. |
| Electrical resistivity—all native metals (gold, copper, silver) and many metalloid (metal-bearing) minerals will conduct electricity. Most metal ore minerals will conduct electricity. Common examples include iron ores: hematite, magnetite, pyrite, chalcopyrite, bornite, galena. Minerals with a metallic luster will conduct electricity. Conversely, non-metallic minerals will not conduct electricity. Quartz and calcite will not conduct electricity. |
 |
| Figure 2-64. A simple electrical resistivity measuring device, shown here, has a battery, a micro-ampere meter, and wires attached to electrodes (nails). This test shows that a sample of bornite (copper "peacock ore") conducts electricity quite well. Parts of a flashlight can be used to make an electrical conductivity testing device. |
Silicate Minerals |
Understanding the nature of silicate minerals is important!Silicate minerals are the dominant group of minerals that make up the rocky crusts of the Earth, Moon, and other stony planets (Mercury, Venus, Mars, and many other moons and asteroids within the Solar System).Silicate minerals chemically consist of compounds that contain the geometric arrangement of silicon-oxide tetrahedrons contained within simple to complex crystalline structures. Other elements combine with the silicon-oxide to form many different minerals with unique physical properties. Mafic vs. Felsic Minerals (and Rocks)The Earth's crust and mantle are dominantly composed of silicate minerals and are commonly associated with varieties of igneous and metamorphic rocks formed in specific geologic settings (and are important to discussion about plate tectonics, discussed in Chapter 7). Common silicate minerals (and the rocks they form) are grouped into two general classes: mafic and felsic.The term mafic refers to silicate minerals (and the rocks they form) that are enriched in the metals of magnesium and iron. Mafic materials (rocks and minerals) tend to be dark colored (Figure 2-65). Mafic minerals include varieties of olivine, pyroxene, and amphibole (illustrated below). Note that there are several varieties of each mafic mineral depending on other elements present in their crystal structures. Mafic rocks are commonly associated with rocks formed on ocean basin settings and are associated with very hot magmas derived from sources in the Earth's mantle. In contrast, the term felsic (named after feldspar) are minerals or rocks rich in silica and aluminum relative to other metals. Felsic minerals (and the rocks they form) tend to be light colored (Figure 2-66). Common felsic minerals include quartz, certain feldspars (including orthoclase, and the sodium-rich plagioclase) and muscovite mica (see below). Felsic rocks are generally associated with rocks formed in continental settings. Molten material that form felsic rocks and minerals typical comes from the melting (or remelting) of preexisting materials and are associated with magma (and lava) that is not nearly as hot as molten material associated with mafic sources. |
 Fig. 2-65. Basalt is a dark colored igneous rock composed of mafic minerals. Basalt is the dominant rock found under ocean basins and exposed in places like Hawaii.  Fig. 2-66. Granite is an igneous rock made up of light-colored felsic minerals, mostly quartz and varieties of feldspar minerals. Granite is found in abundance in the core of continental regions. |
Crystal Structures of Common Silicate MineralsSilicate minerals are the dominant group of minerals that make up the rocky crusts of the Earth, Moon, and other stony planets (Mercury, Venus, Mars, and many other moons and asteroids within the Solar System. Silicate minerals chemically consist of compounds that contain the geometric arrangement of silicon-oxide tetrahedrons contained within simple to complex crystalline structures (Figures 2-67 and 2-68). Silicon-oxide tetrahedrons combine to form chains, sheets, and other complex crystal arrangements. Other elements combine with the silicon-oxide tetrahedrons to form many different minerals with unique physical properties. |
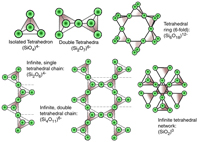 |
 |
| Fig. 2-67. Basic crystalline structure of common silicate minerals. | Fig. 2-68. Structure structure of sheet silicates (including micas and clay minerals). |
Common Silicate Minerals |
||
 |
QuartzQuartz is a hard colorless or white mineral consisting of silicon dioxide (silica-SiO2), found widely in igneous, metamorphic, and sedimentary rocks. Pure silica forms clear quartz crystals in unconfined spaces, such as geodes or open fissures in rock; inclusion of traces of other element in quartz's crystalline structure produces varieties of semiprecious gems varieties including amethyst, citrine, rose quartz, and smoky quartz. Microcrystalline varieties of sedimentary rock composed dominantly of quartz include chert, jasper, flint, agate, and chalcedony. Quartz is a felsic mineral. |
|
| Fig. 2-69. Quartz | ||
 Fig. 2-70. Feldspars |
FeldsparsFeldspars are an abundant rock-forming group of minerals typically occurring as colorless or pale-colored crystals. Feldspars are aluminosilicate minerals with varieties:Orthoclase or K-spar—a variety of feldspar that rich in potassium (KAlSi3O8), Plagioclase—varieties of feldspar rich in feldspar which include sodium-rich Albite (NaAlSi3O8), and calcium-rich Anorthite (CaAlO2SiO2O8). There are many other varieties of feldspars with variable compositions and appearances. Orthoclase and sodium-rich plagioclase is associated with felsic rocks. In contrast, calcium-rich plagioclase forms at higher temperatures than sodium-rich plagioclase and is commonly mixed in with mafic minerals in mafic rocks. |
 |
| Fig. 2-71. Crystal structure of feldspars are combinations of silicon-oxide and aluminum-oxide tetrahedrons with elements of sodium, calcium, potassium and sometimes traces of other elements. | ||
 |
Mafic Silicate MineralsMafic silicate minerals are rich in magnesium and iron. The word mafic is used to describe rocks containing a group of dark-colored, mainly ferromagnesian minerals (rich in iron and magnesium). Mafic rocks are common in the Earth's crust under the ocean basins and are exposed in the volcanoes of Hawaii and Iceland. Mafic and ultramafic rock include such as pyroxene and olivine.olivine—a mineral silicate of iron and magnesium, principally (Mg,Fe)2SiO4, found in igneous and metamorphic rocks occurring in basalt, peridotite, and other basic igneous rocks. pyroxene—Any of a large class of rock-forming silicate minerals, generally containing containing two metallic oxides combining magnesium, iron, calcium, sodium, or aluminum and typically occurring as prismatic crystals. amphibole—Any of a class of rock-forming silicate or aluminosilicate minerals typically occurring as fibrous or columnar crystals consisting of hydrated double silicate minerals, such as hornblende, containing various combinations of sodium, calcium, magnesium, iron, and aluminum. |
|
| Fig. 2-72. Mafic minerals | ||
 Fig. 2-73. Mica minerals |
Micas And Clay MineralsMicas and clay minerals are silicate minerals that have a sheet-like crystal arrangement that allow them to cleave into thin sheets.Micas include: biotite—a common rock-forming mineral occurring in black, dark-brown, or dark -green sheets and flakes: an important constituent of igneous and metamorphic rocks. Biotite is a mafic variety of mica. muscovite—a silver-gray form of mica (platy sheet silicate mineral) occurring in many igneous and metamorphic rocks. Muscovite is a felsic variety of mica. |
 Fig. 2-74. Mica minerals easily peel into thin sheets that are quite flexible. This is related to their sheet-like crystal structure. Fig. 2-74. Mica minerals easily peel into thin sheets that are quite flexible. This is related to their sheet-like crystal structure. |
Clay MineralsClay minerals are any of a group of minerals that occur as microscopic sheet-like or fibrous crystals in clay. Clay minerals are a primary component of many soils and form from the weathering decay of other silicate and aluminum-rich minerals, such as feldspars, micas, and other minerals. Like micas illustrated above, clay minerals have sheet framework crystals.Clay minerals form from the chemical breakdown of other silicate minerals that are not stable in wet surface conditions. Clays themselves are very stable in the surface environment. However, clay minerals will gradually convert back to other silicate minerals when subjected to heat and pressure associated with deep burial and metamorphism. |
 Fig. 2-75. Clay minerals (shown here in microscopic view). |
|
The Rock Cycle RevisitedThe physical and chemical properties of minerals (and the rocks and sediments they form) is essential and fundamental information to understanding all the natural processes taking place on the surface and underground. Minerals are subject to change when subjected to exposure to air or water (particularly gases and fluids beneath the surface)(Figure 2-76). Heat and pressure influence the internal crystal structure and stability of minerals. A mineral that forms miles-deep underground may not be able to withstand the exposure to conditions on the surface. Likewise, some minerals that are stable on the surface environment are altered by heat, pressure, and chemical changes that take place with increasing burial. |
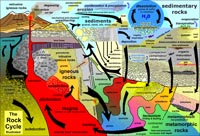 Fig. 2-76. The Rock Cycle Illustrated |